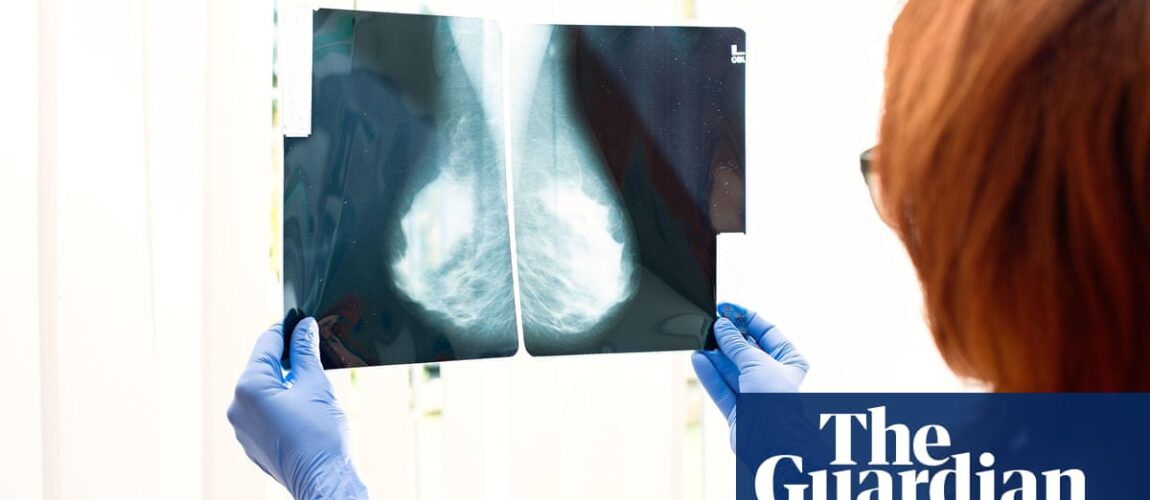
Thousand women with the morning breast cancer could be offered by drug to stop cancer back after drugs watchdog approved use of England.
To 4,000 patients in the year could be given Ribociclib brick hormone loremFor a hormone receptor-positive, her2, negative early breast cancer who despite the initial treatment has a higher risk of returning.
Globally, One in 20 women and develop breast cancer in their lifetimeThe cases up 38% of the deaths up to 68% on the next 25 years, according to the latest analysis of an international agency to research in CANCER (Iarcer).
In the UK, breast cancer rates expected to rise from the fifth to 71,006 cases in the year in 2040. Most common type of breast cancer – accounting for around 70% of cases – are hormone goats.
In National Institute for Health and Care Excellence (Nice) said to the drug, brand brand name Kisqali, it will be available to patients with cancer and spread to nodes of nodes and meets at least one of the following criteria:
-
Cancer present at least four nodes or nodes
-
Cancer present in three nodes of nodes, which is either step 3 (more advanced); or has the primary tumor at least 5cm in size.
Ribociclib shields and blocks proteins called CDK 4 and CDK 6, and play a role in cancer cell growth and division, help to slow or stop tumor growth. Taken to get a pill twice a day, side an aromatase inhibitor, a hormone there reduces estrogen levels in the body.
Clinical trials to show that combining ribociclib with aromatase inhibitor May extend the time before cancer returns to 29%Compared with using an aromatase inhibitor only.
In the decision to follow Nice approval at the April capivasertib To handle advanced hormone receptor (HR) -Positive HER2, negative breast cancer that has spread, which could benefit more than 3,000 women in the year.
But cancer charities said disappointing nice do not approve ribociclib use of those with other forms of the first breast cancer at high risk recurrence.
Claire Rowney, Chief Executive Breast Cancer: “We’re about thousands of people with early breast cancer could miss out to access the vital type of treatment from the uncertain about the cost-effectiveness.
“Despite the Promising Potential for help Ribociclib to Cut the Risk of Cancer Coming Back by almost a third (28.5%), Today’s decision means only certain people with high-risk node positive disease, and none with high-risk node negative disease, would receive it.
“While drug approvals are always nice, it’s disappointing as many people can be denied access to this vital treatment and accident to relieve something anxiety of cancer back.
“Nice to work together to work together to resolve the uncertain uncertain about the cost-effectiveness, such as rapid change to the guidance that everyone who could benefit can receive a help ribococlib.”